 Multiple Exceptions (user mode) - Modeling Example
Multiple Exceptions (user mode) - Modeling Example Multiple Exceptions (kernel mode)
Multiple Exceptions (kernel mode) Multiple Exceptions (managed space)
Multiple Exceptions (managed space)- Multiple Exceptions (stowed)
 Dynamic Memory Corruption (process heap)
Dynamic Memory Corruption (process heap) Dynamic Memory Corruption (kernel pool)
Dynamic Memory Corruption (kernel pool)- Dynamic Memory Corruption (managed heap)
 False Positive Dump
False Positive Dump Lateral Damage (general)
Lateral Damage (general)- Lateral Damage (CPU mode)
 Optimized Code (function parameter reuse)
Optimized Code (function parameter reuse) Invalid Pointer (general)
Invalid Pointer (general)- Invalid Pointer (objects)
 NULL Pointer (code)
NULL Pointer (code) NULL Pointer (data)
NULL Pointer (data) Inconsistent Dump
Inconsistent Dump Hidden Exception (user space)
Hidden Exception (user space)- Hidden Exception (kernel space)
- Hidden Exception (managed space)
 Deadlock (critical sections)
Deadlock (critical sections) Deadlock (executive resources)
Deadlock (executive resources) Deadlock (mixed objects, user space)
Deadlock (mixed objects, user space) Deadlock (LPC)
Deadlock (LPC) Deadlock (mixed objects, kernel space)
Deadlock (mixed objects, kernel space) Deadlock (self)
Deadlock (self)- Deadlock (managed space)
- Deadlock (.NET Finalizer)
 Changed Environment
Changed Environment Incorrect Stack Trace
Incorrect Stack Trace OMAP Code Optimization
OMAP Code Optimization No Component Symbols
No Component Symbols Insufficient Memory (committed memory)
Insufficient Memory (committed memory) Insufficient Memory (handle leak)
Insufficient Memory (handle leak) Insufficient Memory (kernel pool)
Insufficient Memory (kernel pool) Insufficient Memory (PTE)
Insufficient Memory (PTE) Insufficient Memory (module fragmentation)
Insufficient Memory (module fragmentation) Insufficient Memory (physical memory)
Insufficient Memory (physical memory) Insufficient Memory (control blocks)
Insufficient Memory (control blocks)- Insufficient Memory (reserved virtual memory)
- Insufficient Memory (session pool)
- Insufficient Memory (stack trace database)
- Insufficient Memory (region)
- Insufficient Memory (stack)
 Spiking Thread
Spiking Thread Module Variety
Module Variety Stack Overflow (kernel mode)
Stack Overflow (kernel mode) Stack Overflow (user mode)
Stack Overflow (user mode) Stack Overflow (software implementation)
Stack Overflow (software implementation)- Stack Overflow (insufficient memory)
- Stack Overflow (managed space)
 Managed Code Exception
Managed Code Exception- Managed Code Exception (Scala)
- Managed Code Exception (Python)
 Truncated Dump
Truncated Dump Waiting Thread Time (kernel dumps)
Waiting Thread Time (kernel dumps) Waiting Thread Time (user dumps)
Waiting Thread Time (user dumps) Memory Leak (process heap) - Modeling Example
Memory Leak (process heap) - Modeling Example Memory Leak (.NET heap)
Memory Leak (.NET heap)- Memory Leak (page tables)
- Memory Leak (I/O completion packets)
- Memory Leak (regions)
 Missing Thread (user space)
Missing Thread (user space)- Missing Thread (kernel space)
 Unknown Component
Unknown Component Double Free (process heap)
Double Free (process heap) Double Free (kernel pool)
Double Free (kernel pool) Coincidental Symbolic Information
Coincidental Symbolic Information Stack Trace
Stack Trace- Stack Trace (I/O request)
- Stack Trace (file system filters)
- Stack Trace (database)
- Stack Trace (I/O devices)
 Virtualized Process (WOW64)
Virtualized Process (WOW64)- Virtualized Process (ARM64EC and CHPE)
 Stack Trace Collection (unmanaged space)
Stack Trace Collection (unmanaged space)- Stack Trace Collection (managed space)
- Stack Trace Collection (predicate)
- Stack Trace Collection (I/O requests)
- Stack Trace Collection (CPUs)
 Coupled Processes (strong)
Coupled Processes (strong) Coupled Processes (weak)
Coupled Processes (weak) Coupled Processes (semantics)
Coupled Processes (semantics) High Contention (executive resources)
High Contention (executive resources) High Contention (critical sections)
High Contention (critical sections) High Contention (processors)
High Contention (processors)- High Contention (.NET CLR monitors)
- High Contention (.NET heap)
- High Contention (sockets)
 Accidental Lock
Accidental Lock Passive Thread (user space)
Passive Thread (user space) Passive System Thread (kernel space)
Passive System Thread (kernel space) Main Thread
Main Thread Busy System
Busy System Historical Information
Historical Information Object Distribution Anomaly (IRP)
Object Distribution Anomaly (IRP)- Object Distribution Anomaly (.NET heap)
 Local Buffer Overflow (user space)
Local Buffer Overflow (user space)- Local Buffer Overflow (kernel space)
 Early Crash Dump
Early Crash Dump Hooked Functions (user space)
Hooked Functions (user space) Hooked Functions (kernel space)
Hooked Functions (kernel space)- Hooked Modules
 Custom Exception Handler (user space)
Custom Exception Handler (user space) Custom Exception Handler (kernel space)
Custom Exception Handler (kernel space) Special Stack Trace
Special Stack Trace Manual Dump (kernel)
Manual Dump (kernel) Manual Dump (process)
Manual Dump (process) Wait Chain (general)
Wait Chain (general) Wait Chain (critical sections)
Wait Chain (critical sections) Wait Chain (executive resources)
Wait Chain (executive resources) Wait Chain (thread objects)
Wait Chain (thread objects) Wait Chain (LPC/ALPC)
Wait Chain (LPC/ALPC) Wait Chain (process objects)
Wait Chain (process objects) Wait Chain (RPC)
Wait Chain (RPC) Wait Chain (window messaging)
Wait Chain (window messaging) Wait Chain (named pipes)
Wait Chain (named pipes)- Wait Chain (mutex objects)
- Wait Chain (pushlocks)
- Wait Chain (CLR monitors)
- Wait Chain (RTL_RESOURCE)
- Wait Chain (modules)
- Wait Chain (nonstandard synchronization)
- Wait Chain (C++11, condition variable)
- Wait Chain (SRW lock)
 Corrupt Dump
Corrupt Dump Dispatch Level Spin
Dispatch Level Spin No Process Dumps
No Process Dumps No System Dumps
No System Dumps Suspended Thread
Suspended Thread Special Process
Special Process Frame Pointer Omission
Frame Pointer Omission False Function Parameters
False Function Parameters Message Box
Message Box Self-Dump
Self-Dump Blocked Thread (software)
Blocked Thread (software) Blocked Thread (hardware)
Blocked Thread (hardware)- Blocked Thread (timeout)
 Zombie Processes
Zombie Processes Wild Pointer
Wild Pointer Wild Code
Wild Code Hardware Error
Hardware Error Handle Limit (GDI, kernel space)
Handle Limit (GDI, kernel space)- Handle Limit (GDI, user space)
 Missing Component (general)
Missing Component (general) Missing Component (static linking, user mode)
Missing Component (static linking, user mode) Execution Residue (unmanaged space, user)
Execution Residue (unmanaged space, user)- Execution Residue (unmanaged space, kernel)
- Execution Residue (managed space)
 Optimized VM Layout
Optimized VM Layout- Invalid Handle (general)
- Invalid Handle (managed space)
- Overaged System
- Thread Starvation (realtime priority)
- Thread Starvation (normal priority)
- Duplicated Module
- Not My Version (software)
- Not My Version (hardware)
- Data Contents Locality
- Nested Exceptions (unmanaged code)
- Nested Exceptions (managed code)
- Affine Thread
- Self-Diagnosis (user mode)
- Self-Diagnosis (kernel mode)
- Self-Diagnosis (registry)
- Inline Function Optimization (unmanaged code)
- Inline Function Optimization (managed code)
- Critical Section Corruption
- Lost Opportunity
- Young System
- Last Error Collection
- Hidden Module
- Data Alignment (page boundary)
- C++ Exception
- Divide by Zero (user mode)
- Divide by Zero (kernel mode)
- Swarm of Shared Locks
- Process Factory
- Paged Out Data
- Semantic Split
- Pass Through Function
- JIT Code (.NET)
- JIT Code (Java)
- Ubiquitous Component (user space)
- Ubiquitous Component (kernel space)
- Nested Offender
- Virtualized System
- Effect Component
- Well-Tested Function
- Mixed Exception
- Random Object
- Missing Process
- Platform-Specific Debugger
- Value Deviation (stack trace)
- Value Deviation (structure field)
- Runtime Thread (CLR)
- Runtime Thread (Python, Linux)
- Coincidental Frames
- Fault Context
- Hardware Activity
- Incorrect Symbolic Information
- Message Hooks - Modeling Example
- Coupled Machines
- Abridged Dump
- Exception Stack Trace
- Distributed Spike
- Instrumentation Information
- Template Module
- Invalid Exception Information
- Shared Buffer Overwrite
- Pervasive System
- Problem Exception Handler
- Same Vendor
- Crash Signature
- Blocked Queue (LPC/ALPC)
- Fat Process Dump
- Invalid Parameter (process heap)
- Invalid Parameter (runtime function)
- String Parameter
- Well-Tested Module
- Embedded Comment
- Hooking Level
- Blocking Module
- Dual Stack Trace
- Environment Hint
- Top Module
- Livelock
- Technology-Specific Subtrace (COM interface invocation)
- Technology-Specific Subtrace (dynamic memory)
- Technology-Specific Subtrace (JIT .NET code)
- Technology-Specific Subtrace (COM client call)
- Dialog Box
- Instrumentation Side Effect
- Semantic Structure (PID.TID)
- Directing Module
- Least Common Frame
- Truncated Stack Trace
- Data Correlation (function parameters)
- Data Correlation (CPU times)
- Module Hint
- Version-Specific Extension
- Cloud Environment
- No Data Types
- Managed Stack Trace
- Managed Stack Trace (Scala)
- Managed Stack Trace (Python)
- Coupled Modules
- Thread Age
- Unsynchronized Dumps
- Pleiades
- Quiet Dump
- Blocking File
- Problem Vocabulary
- Activation Context
- Stack Trace Set
- Double IRP Completion
- Caller-n-Callee
- Annotated Disassembly (JIT .NET code)
- Annotated Disassembly (unmanaged code)
- Handled Exception (user space)
- Handled Exception (.NET CLR)
- Handled Exception (kernel space)
- Duplicate Extension
- Special Thread (.NET CLR)
- Hidden Parameter
- FPU Exception
- Module Variable
- System Object
- Value References
- Debugger Bug
- Empty Stack Trace
- Problem Module
- Disconnected Network Adapter
- Network Packet Buildup
- Unrecognizable Symbolic Information
- Translated Exception
- Regular Data
- Late Crash Dump
- Blocked DPC
- Coincidental Error Code
- Punctuated Memory Leak
- No Current Thread
- Value Adding Process
- Activity Resonance
- Stored Exception
- Spike Interval
- Stack Trace Change
- Unloaded Module
- Deviant Module
- Paratext
- Incomplete Session
- Error Reporting Fault
- First Fault Stack Trace
- Frozen Process
- Disk Packet Buildup
- Hidden Process
- Active Thread (Mac OS X)
- Active Thread (Windows)
- Critical Stack Trace
- Handle Leak
- Module Collection
- Module Collection (predicate)
- Deviant Token
- Step Dumps
- Broken Link
- Debugger Omission
- Glued Stack Trace
- Reduced Symbolic Information
- Injected Symbols
- Distributed Wait Chain
- One-Thread Process
- Module Product Process
- Crash Signature Invariant
- Small Value
- Shared Structure
- Thread Cluster
- False Effective Address
- Screwbolt Wait Chain
- Design Value
- Hidden IRP
- Tampered Dump
- Memory Fluctuation (process heap)
- Last Object
- Rough Stack Trace (unmanaged space)
- Rough Stack Trace (managed space)
- Past Stack Trace
- Ghost Thread
- Dry Weight
- Exception Module
- Reference Leak
- Origin Module
- Hidden Call
- Corrupt Structure
- Software Exception
- Crashed Process
- Variable Subtrace
- User Space Evidence
- Internal Stack Trace
- Distributed Exception (managed code)
- Thread Poset
- Stack Trace Surface
- Hidden Stack Trace
- Evental Dumps
- Clone Dump
- Parameter Flow
- Critical Region
- Diachronic Module
- Constant Subtrace
- Not My Thread
- Window Hint
- Place Trace
- Stack Trace Signature
- Relative Memory Leak
- Quotient Stack Trace
- Module Stack Trace
- Foreign Module Frame
- Unified Stack Trace
- Mirror Dump Set
- Memory Fibration
- Aggregated Frames
- Frame Regularity
- Stack Trace Motif
- System Call
- Stack Trace Race
- Hyperdump
- Disassembly Ambiguity
- Exception Reporting Thread
- Active Space
- Subsystem Modules
- Region Profile
- Region Clusters
- Source Stack Trace
- Hidden Stack
- Interrupt Stack
- False Memory
- Frame Trace
- Pointer Cone
- Context Pointer
- Pointer Class
- False Frame
- Procedure Call Chain
- C++ Object
- COM Exception
- Structure Sheaf
- Saved Exception Context (.NET)
- Shared Thread
- Spiking Interrupts
- Structure Field Collection
- Black Box
- Rough Stack Trace Collection (unmanaged space)
- COM Object
- Shared Page
- Exception Collection
- Dereference Nearpoint
- Address Representations
- Near Exception
- Shadow Stack Trace
- Past Process
- Foreign Stack
- Annotated Stack Trace
- Disassembly Summary
- Region Summary
- Analysis Summary
- Region Spectrum
- Normalized Region
- Function Pointer
- Interrupt Stack Collection
- DPC Stack Collection
- Dump Context
- False Local Address
- Encoded Pointer
- Latent Structure
- ISA-Specific Code
Artificial Chemistry Approach to Software Trace and Log Analysis
In the past we proposed two metaphors regarding software trace and log analysis patterns (we abbreviate them as TAP):
- TAP as “genes” of software structure and behavior.
- Logs as “proteins” generated by code with TAP as patterns of “protein” structure.
We now introduce a third metaphor with strong modeling and implementation potential we are currently working on: Artificial Chemistry (AC) approach* where logs are “DNA” and log analysis is a set of reactions between logs and TAP which are individual “molecules”.
In addition to trace and logs as “macro-molecules”, we also have different molecule families of general patterns (P) and concrete patterns (C). General patterns, general analysis (L) and concrete analysis (A) patterns are also molecules (that may also be composed of patterns and analysis patterns) that may serve the role of enzymes. Here we follow the division of patterns into four types. During the reaction, a trace T is usually transformed into T’ (having a different “energy”) molecule (with a marked site to necessitate further elastic collisions to avoid duplicate analysis).
T + Pi -> T’ + Pi + Ck
T + Ci -> T’ + 2Ci
T + Li -> T’ + Li + Ck
T + Li -> T’ + Li + Ak
T + Ai -> T’ + Ai + Ak
Ci + Ck -> Ci-Ck
Cl + Cm -> Ck
Ai + Ak -> Ai-Ak
T + Ai-Ak -> T’ + Ai-Ak + Ci
…
Different reactions can be dynamically specified according to a reactor algorithm. The following diagram shows a few elementary reactions:
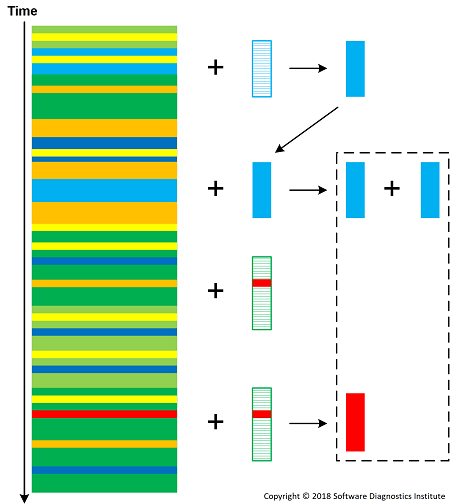
Concentrations of patterns (reaction educts) increases the chances of producing reaction products according to corresponding reaction "mass action". We can also introduce pattern consuming reactions such as T + Li -> T' + Ck but this requires the constant supply of analysis pattern molecules. Intermediate molecules may react with a log as well and be a part of analysis construction (second order trace and log).
Since traces and logs can be enormous, such reactions can occur randomly according to the Brownian motion of molecules. The reactor algorithm can also use Trace Sharding.
Some reactions may catalyze log transformation into a secondary structure with certain TAP molecules now binding to log sites. Alternatively, we can use different types of reactors, for example, well stirred or topologically arranged. We visualize a reactor for the reactions shown in the diagram above:
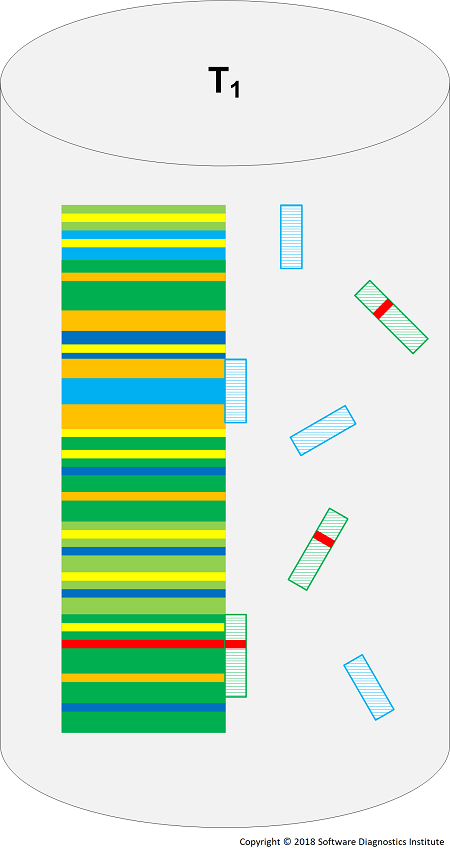

We can also add reactions that split and concatenate traces based on collision with certain patterns and reactions between different logs.
Many AC reactions are unpredictable and may uncover emergent novelty that can be missed during the traditional pattern matching and rule-based techniques.
The AC approach also allows simulations of various pattern and reaction sets independently of concrete traces and logs to find the best analysis approaches.
In addition to software trace and log analysis of traditional software execution artifacts, the same AC approach can be applied to malware analysis, network trace analysis and pattern-oriented software data analysis in general.
* Artificial Chemistries by Wolfgang Banzhaf and Lidia Yamamoto (ISBN: 978-0262029438)